The Baran group published the complete account of their total synthesis of ingenol and it is really worth a read. Also from the same group is an account of the synthesis of the antibacterial Dixiamycin B using an unusual electrochemical oxidation to make a N-N bond leading to a dimeric alkaloid.
Now I must admit that electrochemistry would not have sprung to my mind when faced with this problem, in fact I’m not sure which method I may have used, which is probably why I’m here and not at Scripps!
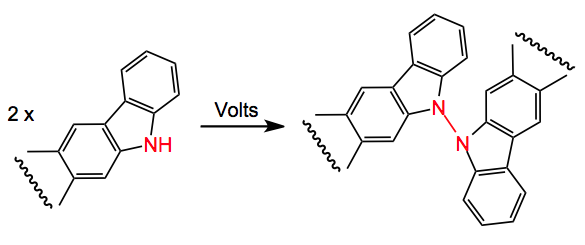
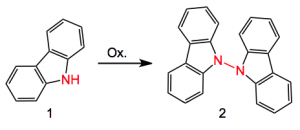
Extending their previous experience with oxidative C-C coupling the question was asked; can this be extended to N-N coupling? Using the carbazole 1 as a model a range of chemical oxidants was examined for their efficacy in the N-N bond formation to give 2.
The usual sort of oxidising agents such as permanganate, LDA then Cu, KOtBu/tBuOCl gave little or no product and in any case these were not expected to be beneficial towards the real substrate! There is precedent for electrochemical oxidation in organic synthesis and a review but the latest reference is 2008, so it is not very common. An examination of this method for the synthesis of 2 produced the following conditions: DMF/5%MeOH, 18hr, carbon anode, 1.2 volts Ag/AgCl and tetraethyl ammonium bromide. This gave a 63% yield of compound 2. Using 1.6 volta resulted in decomposition, so the exact control of the oxidation potential is a prerequisite for success. Various substituted carbazoles were also examined and the corresponding N-N dimers were obtained in low to good yields. Epoxides, C=C and pyridines are all well tolerated and survived the voltage.
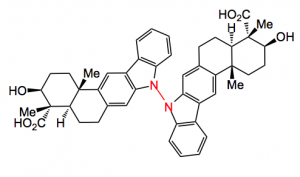
Xiamycin was synthesised by a literature procedure and electrochemically oxidised. The desired compound was obtained in 28% yield along with 13% recovered starting material and 17% of a brominated product (aromatic bromination). A different phase transfer catalyst should fix that, in my view.
So another valuable contribution from this group hits the presses. Congratulations to all, especially those doing all the hard work in the lab. It’s not often that we see a new method these days.
3,678 total views, 1 views today