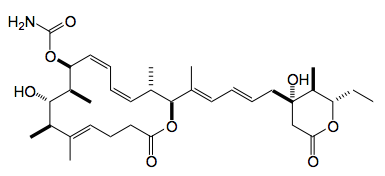
This week Fürstner working together with a group from Pfizer published a full paper on the total synthesis and biological activity of the marine natural product leiodermatolide:-
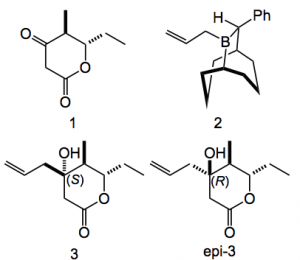
This paper is so full of interesting chemistry it’s difficult to know where to start! Anyway it is a refinement and scale up of the first route to this compound reported by Fürstner. This was deemed satisfactory however some fine tuning was required. For example the synthesis of the δ-lactone required attention, not for uncertainties in the structure but to unambiguously be satisfied that one can obtain the key intermediate in a way that allows it’s stereochemistry to be exactly controlled. This is especially relevant as the chain attaching the lactone to the rest of the molecule is axially orientated. The original approach to the required building block relied upon a reagent-controlled allylation of the cyclic β-ketoester (1), with the chiral boron reagent (2) to give the allyl alcohol (3) and epi (3) in good yields and reasonable dr. (5.5:1 in favour of the (S) diastereoisomer):
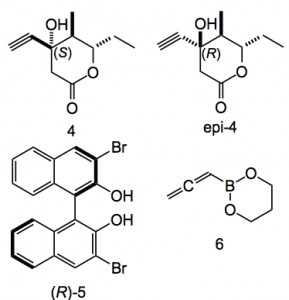
This result was only achieved after considerable experimentation one of the reasons being the ready enolisation of (1). This was carried out en-route to a Suzuki fragment coupling. The problem(s) was that stoichiometric amounts of the 9-BBN analogue were required and the cross-coupling was difficult. Past results demonstrated that an adaption of the Stille coupling performed better in this particular case especially when polyunsaturated and/or base-sensitive compounds are used. Thus the allylation step was replaced by a catalytic propargylation step producing (4) and epi (4) using the chiral catalyst (5) together with boronate (6).
Note that here the catalyst amount is around 10 mol% and microwave heating is used to provide (4) in 94% yield with a dr of 7.6:1. Also gram amounts of (4) were produced.
From the authors:”Leiodermatolide (1) was thus procured in 6% overall yield over the 19 steps of the longest linear sequence. Importantly, all supply limiting transformations of the first generation synthesis have been replaced by robust alternatives; in this context, the catalytic asymmetric propargylation of the enolisable β-keto- lactone and the Stille coupling in lieu of the Suzuki reaction denote particularly substantial improvements. Equally note- worthy is the fact that two solutions for the RCAM step were found once it had been recognised that the original difficulties in closing the macrocyclic frame were steric in origin. The new route allows gram quantities of all building blocks to be made without undue efforts. If elaborated as described above, substantial amounts of the natural product come into reach.”
They prepared 50 mg of leiodermatolide however the actual material on the shelf would have provided some 500 mg. They decided to retain material primarily to investigate the SAR of this molecule and a number of structures were synthesised in order to identify the pharmacophore. Two analogues were identified with significant activity towards a panel of human cancer cell lines.
I enjoyed reading this paper, it’s full of bits of valuable information, much more that I can do justice to here. So go have a good read and learn from the master himself.
2,230 total views, 1 views today