Following on from styrene production with the new rhodium catalyst is this week’s ASAP, still dealing with styrene, but this time making it from glucose, not benzene. In the area of synthetic biology Balskus & Wallace from Harvard report a biocompatible iron(III) phthalocyanine catalyst capable of efficient olefin cyclopropanation and can be employed within a living system.
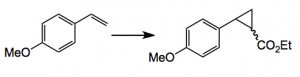
So the basic idea is “the engineering of nonbiological reactivity into enzymes and the development of non-enzymatic catalysts that can be interfaced with cellular metabolism.” The non-enzymatic reaction in this case is the cyclopropanation reaction of styrenes with diazoacetates catalysed by the iron(III) system. In order for this to work reaction conditions must be chosen that are compatible with keeping the cellular metabolism going. The cellular metabolism system is that of E. coli. Conditions were found for the following reaction:
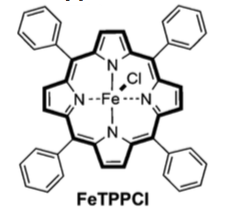
Conditions: 5 mmol vinyl anisole, 10 mmol EDTA, 0.5 mmol Fe catalyst, M9Ca-glucose growth media, E. coli, aerobic, 37°C, 18 hr. This produces the cyclopropane in 75% yield with a trans/cis ratio of 4/1. The catalyst is FeTPPCl.
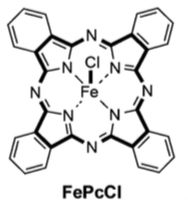
Other catalysts were screened and the FePcCl (10 mol% or 1 mol% for 48 hr) was found to be superior. The yield of cyclopropane went up to 95% with similar trans/cis ratios. Interestingly “The major by-product of the reaction at a catalyst loading of 10mol% was diethyl maleate, which arises from EDA dimerisation. Unexpectedly, at lower catalyst loadings we observed a new by-product, diethyl succinate”.
Styrene production itself was generated by bacterial metabolism from D-glucose using an E.coli strain which overproduces L-phenylalanine after the addition of two enzymes “L-phenylalanine ammonia lyase from Arabidopsis thaliana (PAL2), which converts l-phenylalanine into trans-cinnamic acid, and a decarboxylase from Saccharomyces cerevisiae (FDC1) that generates styrene from trans- cinnamic acid.“
So the poor E.coli was once again genetically modified with the genes coding for these two enzymes and obligingly it produced 1.65 mmol of styrene within 48 hours. Cyclopropanation proceeded as expected.
In summary from the authors:”we have shown that biocompatible metallocarbene-transfer catalysis and engineered metabolism can be combined for small-molecule production. The integration of metallocarbene chemistry with the metabolism of living organisms is a new approach to the construction of non- natural molecules.”
Now folks, tell me which is more sustainable chemistry for the production of styrene; the rhodium approach of the glucose route? I know which one I would pick. Feeding glucose to E.coli must be like opening the dairy to a cat! This paper makes the Science publication redundant.
8,020 total views, 2 views today