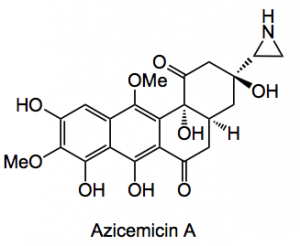
Several things caught my eye this week so a brief synopsis of each of the more interesting ones. A nice review of the stereoselective synthesis of aziridines by Dengennaro, Trinchera and Luisi from the University of Bari. There are quite a few natural products containing an aziridine ring for example the mitomycin family and the aptly named azicemicins:
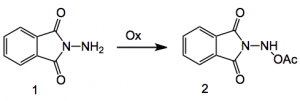
One method caught my eye namely the aziridination using N-aminophthalimide 1 as the nitrene source. This compound upon oxidation with iodobenzenediacetate or electrochemically generates the species 2 which is the active reagent:
This review is certainly worth a read.
The next paper is a process one dealing with the scale-up of a replacement for Micafungin with the inspiring name of ASP9726 emanating from the labs of Astellas Pharma. Unfortunately the molecule is too large to fit in here so here is the smiles:
O[C@@H]([C@@H](C(N[C@@H]([C@H](O)CCNC(CO)CO)C(N1[C@H](C(NC[C@@H](C[C@H](N[C@H]2CC[C@H](C3=NN=C(C4=CC=C(N5CCC(C6CCCCC6)(OC)CC5)C=C4)S3)CC2)C(N[C@@H]([C@@H](C)O)C(N7[C@@H]8C[C@@H](O)C7)=O)=O)O)=O)[C@@H](O)[C@@H](C)C1)=O)=O)NC8=O)CC9=CC=C(O)C(OC)=C9
Or the InChiKey if you prefer: LQHSCHRNMYOZCX-YETSEANXSA-N. Basically it is a cyclic peptide with “bits” hanging off various ends and is an anti-fungal compound. It’s amazing just what these cyclic peptides will stand up to chemically. For example: dehydration of a carboxamide to a nitrile was accomplished by use of EDC·HCl with pyridine. Hydrogenation of the nitrile over sponge nickel catalyst to a primary amine. Reductive aminations just to mention a few. They obtained 66 kg of the peptide core unit and were able to make highly pure ASP9726 without using silica gel or ODS column chromatography purification in any step. Better the overall yield was drastically increased from 0.71% to 13.8% compared to that of the prior synthetic method. Congratulations to this group for an excellent job.
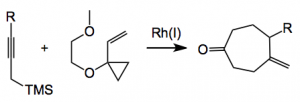
Paul Wender at Stanford has has an interest in higher order cycloadditions for some time now, his latest offering discusses the use of propargyltrimethylsilanes as allene equivalents in Rh catalysed [5+2] cycloadditions as below:
Catalyst screening revealed a good system for this reaction; 1 mol% of [Rh(C10H8)-COD)]SbF6 in dichloroethane (0.15M) at rt for 1 hour gives the cycloaddition product in 98% yield before protodesilylation. A number of functional groups are tolerated with this catalyst for example phthalimido, and benzoic acid esters. So the above reaction can be run in one pot by a short exposure of the cycloadduct to HCl/in ethyl acetate producing the heptanone in 92% yield within 15 minutes reaction time. As the authors say “Significantly, the propargyl- trimethylsilanes not only work as synthetic equivalents of gaseous or otherwise difficult to handle allenes but also enable access to a product that is derived from chemoselective cycloaddition to the more hindered allene double bond. This strategy provides simple, safe, and efficient access to previously inaccessible allene [5 + 2] cycloadducts and has utility for other cycloadditions.” And I can’t add to that statement. Nice work here with plenty of application, especially in Wender’s “step-economical” synthetic routes.
3,377 total views, 1 views today