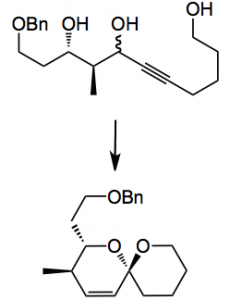
This week I noticed sone gold chemistry from the Aponick group, at the University of Florida, who reported a useful procedure for controlling the regiochemistry during the synthesis of unsaturated spiroketals.
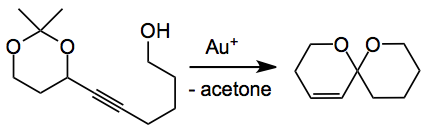
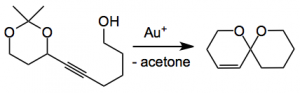
Spiroketals are a structural unit present in many natural products and their synthesis usually constitutes a challenging problem in the grand synthetic scheme. Of course there are many ways to prepare this structural unit. One of the routes involves the spiroketalisation of alkynediols a process that is catalysed by many metals. However, these reactions generally give mixtures of spiroketals due to regioselectivity issues. Now with this new work of Aponick we have a way round this thorny problem which seeks to control the rate of cyclisation leading to the desired product using the following generalised reaction:
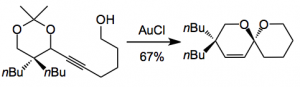
It turns out to be quite facile when the acetylene is treated with a gold(I) complex. The best catalyst was AuCl at a 10 mol% level where the reaction is carried out in THF at 0.1M although the choice of the initial substrate, according to the authors, was not actually the best one and the catalyst loading was later reduced to 5 mol%:
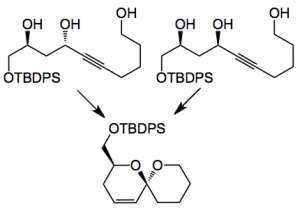
A comparison of various triols and their corresponding acetonides was carried out. This demonstrated, as expected, that the stereochemistry of the triol is vital in obtaining a useful yield of the spiroketal:
The anti triol gave the spiroketal in 85% yield and a dr >25:1 while the syn isomer gave 30% yield with the same dr. With the corresponding acetonides anti gave 72% and syn 74% with the same dr of >25:1. So now with the acetonides the substrate stereochemistry is not important for a good yield. An interesting result is the following:
This is the stereochemistry found in the spirostrellolides. The diol cyclised in 10% yield and the corresponding acetonide in 52%, both giving the spiroketal in 16:1 dr.
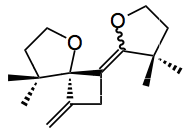
The authors go on to suggest a catalytic cycle for this reaction involving addition of gold to the triple bond followed by cyclisation to produce the first sis-memebered ring. Loss of acetone follows sparking off a rearrangement to a vinyl gold species via an alleneic gold complex. This was tested experimentally and the following compound isolated from the studies:
This is most likely formed by a gold catalysed [2+2] cycloaddition of an allenyl ether. This is not without literature precedent.
So we have here a very useful addition to the synthetic toolbox allowing the synthesis of lots of spiroketals. I’m sure it will find it place in natural product synthesis. A nice piece of work and congratulations to the Aponick group. A nice anniversary present at the end of his first year of tenure.
2,961 total views, 1 views today