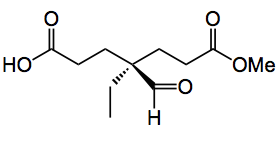
Apart from synthesising your target molecule there is nothing more pleasing than the desymmetrisation of a symmetrical molecule producing a useful chiral synthon. This week’s offering does just that. Described by the group of Jieping Zhu at the ETH Lausanne they synthesised the chiral fragment shown below.
This compound was first prepared by Kuehne and used as a monoterpene equivalent in the synthesis of various vinca alkaloids notably vincamine.
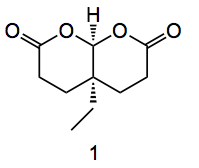
So how was the desymmetrisation mentioned above to give the aldehyde carried out? Well “in the presence of a catalytic amount of chiral phosphoric acid, bicyclic bislactones derived from diesters of the acid above are readily desymmetrized to enantioenriched monoacids having an all-carbon stereogenic center .” After considering various symmmetrical starting materials the ETH group chose the bicyclic lactone 1:
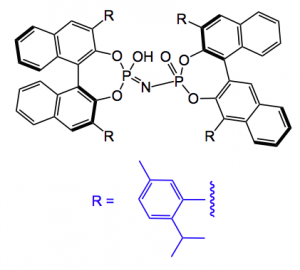
This is easily prepared from the acid by treatment with NaOH and acetic anhydride in high yield. The cis ring junction was confirmed by x-ray structure analysis. There are several ways to desymmetrise this molecule, enzymatically, using a chiral amine catalyst such as an alkaloid, but the group found that use of a chiral phosphoric acid was optimal in this case. The best catalyst was found to be the imidodiphosphoric acid shown below:
This compound was described by List etal. to be an excellent catalyst for the catalytic sulfoxidation. Careful optimisation of the reaction conditions provided the chiral Kuehne aldehyde in a 92:8 enantiomeric ratio in favour of the stereochemistry shown above. The conditions were 1,4-dioxane at RT, with 2 equivalents of an alcohol (methanol in the case above), the reaction time is not mentioned. The concentration of the catalyst was 0.1 equivalents. The yield of the aldehyde was 95%.
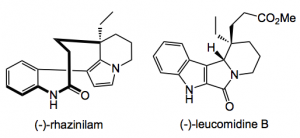
The group then went on to synthesise the alkaloids (-)-rhazinilam and the first total synthesis of (-)-leucomidine B in several steps and good yield.
An additional advantage of this catalyst is its easy removal from the reaction mixture. This work represents a new approach for desymmetrisation of prochiral esters and the painstaking investigation to derive the optimal reaction conditions are a highlight in this paper. So a nice addition to the toolkit of organic chemists. Congratulations.
3,524 total views, 2 views today