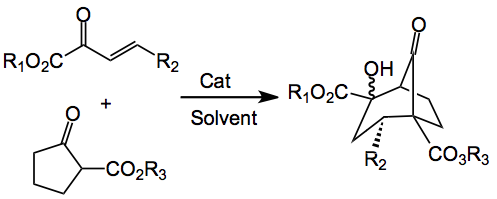
Bicyclo[3.2.1] octanes are an important structural unit that is present in many natural products, as a recent review highlights. Good control of the various stereocenters and the quaternary carbons is of extreme relevance. A new way to achieve this was recently reported by Alexakis et al from the University of Geneva. It is as follows:
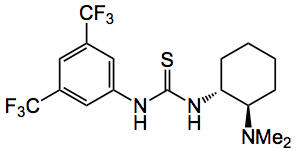
There are probably a few organocatalysts that will catalyse this type of reaction the trick is to find one that is readily available and gives high chemical and optical yields. They began by investigating bifunctional Brønsted acid/base catalysts for example chiral molecules bearing a thiourea and tertiary amine moiety, such as cinchona alkaloid derivatives. The one that proved the most optimal was the following compound described by Takemoto who observed that both the thiourea and the tertiary amine are required for high yields and selectivities :
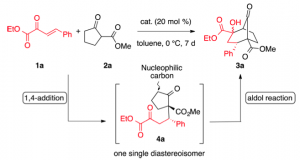
So using 10 mol% of this catalyst, in toluene produced the bicyclic compound in 89% isolated yield with only two of the 4 possible diastereoisomers being produced in a 1.2:1 dr, with the er being 87:13 and 82:18. This is the graduate student’s dream reaction, as it runs for 7 days at 0°C.
The effect of the various substituents was examined. R2 was set as phenyl. R1 can be anything but H, the authors investigated Me, Et, and iPr. For R3 only Me, Et and allyl esters were chosen. For some reason the R1 = R3 = Et gave the lowest yield, 55%, with the allyl ester providing 78%. It would have been nice to see a few more esters used here, benzyl perhaps or even p-methoxy benzyl.
The R2 groups were a variety of substituted aromatics ranging from electron neutral to electron rich to electron deficient aromatic rings. Ortho methoxy and ortho nitrophenyl gave, predictably, the lowest yields, about 55%. One heterocyclic was investigated and styrene gave >95%. These yields being those of the isolated product. A mechanism was proposed:
I think it’s what one would expect to happen. Note in the mechanism the catalyst amount has suddenly increased to 20 mol%. The Michael adduct was isolated after 1 day at 0°C with excellent diastereoselectivity, >20:1 and high er, 90:10.
It would have been nice to see the effect of having chiral esters or a chiral cyclopentanone on this system. It may be possible to match the stereochemistry. Perhaps this will be the subject of future publications. However, here is a good, easy to do robust reaction leading to an important carbon skeleton.
2,207 total views, 1 views today