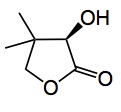
This week we have a paper which takes another look at the synthesis of (R)-pantolactone and comes up with a chemoenzymatic three-step process to this important chiral building block. Incidentally it costs about £1.5/g from Aldrich.
The industrial manufacture relies upon an aldol reaction with formaldehyde followed by cyanohydrin formation, with HCN and a crystallisation of diastereoisomers using chiral auxiliaries,quinine being the favoured one. So not a really ideal process. Enzymatic resolution is also extensively used. However the other, (S)-enantiomer, is re-cycled, adding to the effort and cost.
Berkessel, Gröger and co-workers decided to investigate the idea shown below starting from isobutyraldehyde and glyoxalate:
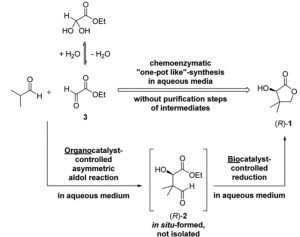 The key intermediate (R)-2 is the product of an asymmetric histidine-catalysed cross-aldol reaction yielding aldehyde (R)-2 in 60% with 65% ee. This process already known to the authors was subjected to intense scrutiny. Obviously the catalyst is the first place to start and they went through lots of amino acid organocatalysts, however none proved to be better than histidine. Reaction time was shown to be optimal at 24 hours. The amount of water was critical only for the conversion, not the ee%. Temperature obviously had an effect on reaction rate and interestingly the ee% was at a maximum at 10°C. The stoichiometry of the reaction was not a crucial parameter.
The key intermediate (R)-2 is the product of an asymmetric histidine-catalysed cross-aldol reaction yielding aldehyde (R)-2 in 60% with 65% ee. This process already known to the authors was subjected to intense scrutiny. Obviously the catalyst is the first place to start and they went through lots of amino acid organocatalysts, however none proved to be better than histidine. Reaction time was shown to be optimal at 24 hours. The amount of water was critical only for the conversion, not the ee%. Temperature obviously had an effect on reaction rate and interestingly the ee% was at a maximum at 10°C. The stoichiometry of the reaction was not a crucial parameter.
As the reaction proceeds via an enamine intermediate the effect of acid was also studied. Applying equimolar amounts of organocatalyst and acid additive, the best results in terms of accelerating the reaction as well as improving optical purity of (R)-2 are found with cocatalysts that have pKa-values between 4 and 5, i.e. benzoic, acetic, pivalic acids. These all accelerated the reaction and improved the ee%. Other organocatalysts such as sulfonamides, amino acids, alkaloids, and squareamides), revealing bifunctional primary-amine thiourea organocatalysts as further suitable catalysts. Urea catalysts based on (R,R)-diaminocyclohexane, for example
gave a 95% conversion with 81 ee%. However mindful of costs “Although preparation of trifluoromethyl organocatalyst is just one step from commercially available starting materials, after detailed evaluation of the potential of all the applied organocatalysts, we decided to use the natural amino acid L-histidine as the organocatalyst of choice for further process optimisation due to its direct commercial availability, attractive price, and similar enantioselectivities (75% ee), which were obtained in the asymmetric aldol reaction for synthesis of (R)-2 when using this natural L-amino acid.” This intensive and exhaustive (if not exhausting) piece of work led to the following conditions for the formation od the crossed-aldol product:
In keeping with this catalytic effort the obvious choice for the reduction/cyclisation final step was enzymatic and they found an alcohol dehydrogenase which at pH 8 or so gave a 67% conversion and 95% ee. After more and more optimisation “we were pleased to find that when removing all volatile materials from the reaction mixture of the aldol reaction prior to its use for biotransformation, conversion is in the same range as that of the benchmark reaction. This illustrates that a workup and purification of intermediate (R)-2 is not required and that simple evaporation of the volatile materials after conducting the organocatalytic transformation is sufficient for a subsequent efficient biocatalytic reduction process.” It should be noted that when isobutyraldehyde and /or acetone are present a big drop in conversion was observed. So these were removed by continuously passing a mixture of air saturated with water/isopropanol through the enzymatic reaction mixture which alters the equlibrium towards the product side and has the appealing effect of reducing the amount of enzyme required. So wrapping all this up the authors achieved a 55% yield over three steps producing (R)-pantolactone in 95% ee.
Here again we find a excellent piece of experimental work and I think that most of this should be somewhat applicable to other enzymatic reactions, especially displacing the equlibrium of the alcohol dehydrogenase step towards product by continuous removal of competing reaction products is always an elegant way of doing things. Lots of scale-up potential here with relatively benign systems. So for all you chemists out there trying to use enzymes, this paper is a must read.
2,468 total views, 1 views today