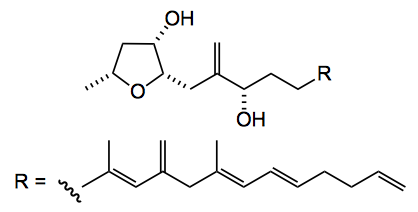
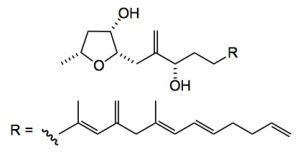
This week sees another total synthesis of a dino. natural product by Britton from the Simon Frazer University in lovely Vancouver. Amphirionin-4, is a trisubstituted tetrahydrofuran with a complex alky/alkene side chain:
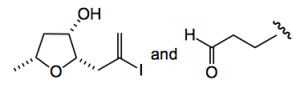
The disconnections are reasonably obvious; various cross-couplings for the side chain polyene and interestingly a NHK reaction to generate the allylic alcohol, thus providing the next two fragments:
The THF unit can then be prepared using Britton’s previous methodology which required enantioenriched α-chloroaldehydes.
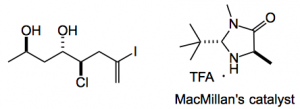
So the synthesis is relatively straightforward from 4-pentyn-1-ol, hydroiodination of the alkyne and a Swern produces the vinyl iodide in 35% yield for the 2 steps, author’s note “Notably, the modest yield for the hydroiodination (46%) reflects the necessary stoppage of this reaction at ∼60% conversion, which was required to prevent the generation of an inseparable byproduct produced after prolonged reaction times (yield >70% yield based on unreacted alkyne.” This is then chlorinated using NCS and MacMillan’s catalyst to give the α-chloroaldehyde which is then an aldol partner with acetone followed by 1,3 anti-selective reduction to give the chloro diol:
The alpha chlorination step went in 90% ee and 61% yield consequently the aldol produced a 7:1 ratio of diastereoisomers. Microwaving this chloro diol at 120°C in methanol for 3 hours gave the desired tetrahydrofuran in 72% yield of course with the desired stereochemistry!
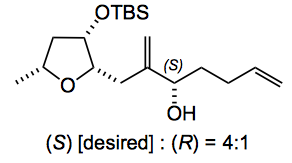
Next up is the NHK reaction. Interestingly they were able to have a decent measure of stereocontrol in this reaction with a model aldehyde and obtained the requisite alcohol along with its stereoisomer in a 4:1 excess when the THF alcohol was protected as a TBS ether:
I brushed off the synthesis of the polyene side chain as a series of cross-couplings! However, upon reading further this actually seemed to me to be more challenging than the THF part.
Several approaches were examined towards the alkyne sub-unit
were examined. Ultimately the vinyl trifluoroborate/Molander chemistry proved to be successful and gave the alkyne in 68% yield. This was converted to
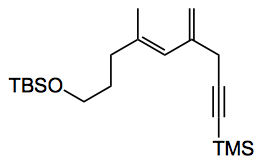
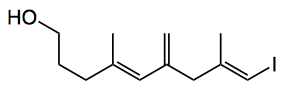
by carboalumination and reaction with iodine in 54% yield. Stille coupling with (E)-tributyl(hexa-1,5-dien-1-yl)stannane finally gave the desired polyene side chain. NHK coupling gave the expected good selectivity. “Unfortunately, all attempts to remove the TBS protecting group from this material resulted in decomposition. Considering the instability of the skipped tetraene function, we elected to modify the synthetic plan and thus incorporate the sensitive tetraene at the final stage in the synthesis“.
Problems were still encountered, even with this approach, “but Fürstner’s conditions, which are ideal for sensitive substrates, proved effective and yielded access to amphirionin-4 in excellent yield over these final two steps“.
So here we have a really good piece of work. It shows that these metal catalysed cross coupling reactions are by no means trivial. The efforts reported here are a good addition to the vast amount of literature on this chemistry. Congratulations.
2,326 total views, 1 views today