Here is a nice route to tetrahydropyridines, to quote the authors: “These products can be accessed on a gram scale with low catalyst loadings and at high reaction concentrations. Additionally, a modified Rh-catalyst, prepared from [RhCl(cod)]2 as a robust bench-stable pre-catalyst was developed to enable straightforward reaction set up without the use of a glovebox.”
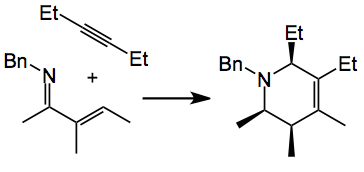
This comment appeared in OPRD and was submitted by Ellman at Yale university. So this statement caught my eye, no glovebox. This makes the use of such catalysts much more attractive for process scale-up. An example of the reaction studied was the following:
The conditions are; 1.5 equiv. acetylene, Rh pre-catalyst, 1 mol%, 4- (diethylphosphino)-N,N-dimethylaniline, 0.5 mol%, 1.5 M in toluene at 80°C, 24 hours, followed by reduction with NaBH(OAc)3. This gave the tetrahydropydidine in 93% yield. Now using the glove box with 0.25 mol% of [RhCl(coe)2]2 nnnn, coe = cyclooctene produced a 95% yield on a 100 mmol scale. The air stable catalyst [RhCl(cod)]2 at 1 mol% produced a 93% yield.
A requirement is a “pre-activation” of the [RhCl(cod)]2 with 4- (diethylphosphino)-N,N-dimethylaniline for 1 hour. A lower catalyst loading was examined however the reaction required a higher temperature and the yield dropped. But a 1 mol% loading is not bad at all.
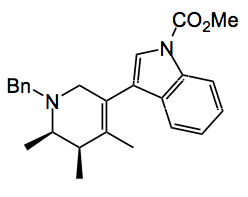
So what can you do with this very interesting system? You stick an interesting group on one side of the acetylene and TMS on the other and you get:
something which looks like it may be biologically relevant.
What does the borohydride do? It reduces the dihydropyridine intermediate from the cycloaddition reaction. This actually turns out to quite tricky and the correct order of addition of the various components is vital. From the authors “To obtain the product of kinetic protonation, it is essential that the iminium only be generated in the presence of the reductant to prevent equilibration to the thermodynamically more stable conjugated iminium isomer. This requires that the dihydropyridine be added to excess NaBH(OAc)3 in ethanol followed by addition of acetic acid.” A silica-gel filtration is necessary to remove rhodium residues.
This is an important development and should be suitable for even larger scale reactions. I wonder what else the catalyst can do? So some nice usable chemistry here, congratulations.
2,376 total views, 1 views today